
The Science of Heavy Metal Detox
The Science of Heavy Metal Detox Natural Approaches to Cleansing Your Body In our modern world, exposure to heavy metals chlorella detox is practically unavoidable. Whether it’s through the food
Introduction
The term heavy metal refers to any metallic chemical element that has a relatively high density and is toxic or poisonous at low concentrations. Heavy metal toxicity is when these metals cause toxicity symptoms in living organisms.
Examples of heavy metals include mercury (Hg), cadmium (Cd), arsenic (As), chromium (Cr), thallium (Tl), and lead (Pb).
Heavy metals are natural components of the Earth’s crust. They are difficult to destroy or decay. They can enter our bodies via food, drinking water and air. Heavy metal toxicity could result, for instance, from drinking-water contamination (e.g. lead pipes), high ambient air concentrations near emission sources, or intake via the food chain.
Heavy metals are dangerous because they tend to bioaccumulate. Bioaccumulation means an increase in the concentration of a chemical in a biological organism over time, compared to the chemical’s concentration in the environment. Compounds accumulate in living things any time they are taken up and stored faster than they are broken down (metabolized) or excreted.
Heavy metals can enter a water supply by industrial and consumer waste, or even from acidic rain breaking down soils and releasing heavy metals into streams, lakes, rivers, and groundwater.
Now we are going to describe the effects of heavy metals on the environment. The three most pollutant heavy metals are Lead, Cadmium, and Mercury.
Antimony is a metal used in the compound antimony trioxide, a flame retardant. It can also be found in batteries, pigments, ceramics, and glass. Exposure to high levels of antimony for short periods of time causes nausea, vomiting, and diarrhea. There is little information on the effects of long-term antimony exposure, but it is a suspected human carcinogen. Most antimony compounds do not bioaccumulate in aquatic life.
Cadmium derives its toxicological properties from its chemical similarity to zinc an essential micronutrient for plants, animals, and humans. Cadmium is persistent and, once absorbed by an organism, remains resident for many years (over decades for humans) although it is eventually excreted.
In humans, long-term exposure is associated with renal dysfunction. High exposure can lead to obstructive lung disease and has been linked to lung cancer, although data concerning the latter are difficult to interpret due to compounding factors. Cadmium may also produce bone defects (osteomalacia, osteoporosis) in humans and animals. In addition, the metal can be linked to increased blood pressure and effects on the myocardium in animals, although most human data do not support these findings.
The average daily intake for humans is estimated as 0.15µg from air and 1µg from water. Smoking a packet of 20 cigarettes can lead to the inhalation of around 2-4µg of cadmium, but levels may vary widely.
Cadmium is produced as an inevitable by-product of zinc (or occasionally lead) refining since these metals occur naturally within the raw ore. However, once collected the cadmium is relatively easy to recycle.
The most significant use of cadmium is in nickel/cadmium batteries, as rechargeable or secondary power sources exhibiting high output, long life, low maintenance, and high tolerance to physical and electrical stress. Cadmium coatings provide good corrosion resistance, particularly in high-stress environments such as marine and aerospace applications where high safety or reliability is required; the coating is preferentially corroded if damaged. Other uses of cadmium are as pigments, stabilizers for PVC, in alloys, and electronic compounds. Cadmium is also present as an impurity in several products, including phosphate fertilizers, detergents, and refined petroleum products.
In the general, non-smoking population the major exposure pathway is through food, via the addition of cadmium to agricultural soil from various sources (atmospheric deposition and fertilizer application) and uptake by food and fodder crops. Additional exposure to humans arises through cadmium in ambient air and drinking water.
Chromium is used in metal alloys and pigments for paints, cement, paper, rubber, and other materials. Low-level exposure can irritate the skin and cause ulceration. Long-term exposure can cause kidney and liver damage, and damage too circulatory and nerve tissue. Chromium often accumulates in aquatic life, adding to the danger of eating fish that may have been exposed to high levels of chromium.
Copper is an essential substance to human life, but in high doses it can cause anemia, liver and kidney damage, and stomach and intestinal irritation. People with Wilson’s disease are at greater risk for health effects from overexposure to copper. Copper normally occurs in drinking water from copper pipes, as well as from additives designed to control algal growth.
In humans, exposure to lead can result in a wide range of biological effects depending on the level and duration of exposure. Various effects occur over a broad range of doses, with the developing foetus and infant being more sensitive than the adult. High levels of exposure may result in toxic biochemical effects in humans which in turn cause problems in the synthesis of haemoglobin, effects on the kidneys, gastrointestinal tract, joints, and reproductive system, and acute or chronic damage to the nervous system.
Lead poisoning, which is so severe as to cause evident illness, is now very rare indeed. At intermediate concentrations, however, there is persuasive evidence that lead can have small, subtle, subclinical effects, particularly on neuropsychological developments in children. Some studies suggest that there may be a loss of up to 2 IQ points for a rise in blood lead levels from 10 to 20µg/dl in young children.
The average daily lead intake for adults in the UK is estimated at 1.6µg from air, 20µg from drinking water, and 28µg from food. Although most people receive the bulk of their lead intake from food, in specific populations other sources may be more important, such as water in areas with lead piping and plumbosolvent water, air near the point of source emissions, soil, dust, paint flakes in old houses or contaminated land. Lead in the air contributes to lead levels in food through the deposition of dust and rain containing the metal, on crops and the soil. For the majority of people in the UK, however, dietary lead exposure is well below the provisional tolerable weekly intake recommended by the UN Food and Agriculture Organisation and the World Health Organisation.
Lead in the environment arises from both natural and anthropogenic sources. Exposure can occur through drinking water, food, air, soil, and dust from old paint containing lead. In the general non-smoking, adult population the major exposure pathway is from food and water. Food, air, water, and dust/soil are the major potential exposure pathways for infants and young children. For infants up to 4 or 5 months of age, air, milk formulae, and water are significant sources.
Lead is among the most recycled non-ferrous metals and its secondary production has therefore grown steadily in spite of declining lead prices. Its physical and chemical properties are applied in the manufacturing, construction, and chemical industries. It is easily shaped and is malleable and ductile. There are eight broad categories of use: batteries, petrol additives (no longer allowed in the EU), rolled and extruded products, alloys, pigments and compounds, cable sheathing, and shot and ammunition.
Mercury is a toxic substance that has no known function in human biochemistry or physiology and does not occur naturally in living organisms. Inorganic mercury poisoning is associated with tremors, gingivitis, and/or minor psychological changes, together with spontaneous abortion and congenital malformation.
Monomethylmercury causes damage to the brain and the central nervous system, while foetal and postnatal exposure has given rise to abortion, congenital malformation, and development changes in young children.
Mercury is a global pollutant with complex and unusual chemical and physical properties. The major natural source of mercury is the degassing of the Earth’s crust, emissions from volcanoes, and evaporation from natural bodies of water.
Worldwide mining of the metal leads to indirect discharges into the atmosphere. The usage of mercury is widespread in industrial processes and in various products (e.g. batteries, lamps, and thermometers). It is also widely used in dentistry as an amalgam for fillings and by the pharmaceutical industry. Concern over mercury in the environment arises from the extremely toxic forms in which mercury can occur.
Mercury is mostly present in the atmosphere in a relatively unreactive form as a gaseous element. The long atmospheric lifetime (of the order of 1 year) of its gaseous form means the emission, transport, and deposition of mercury is a global issue.
Natural biological processes can cause methylated forms of mercury to form which bioaccumulate over a million-fold and concentrate in living organisms, especially fish. These forms of mercury: monomethyl mercury and dimethylmercury are highly toxic, causing neurotoxicological disorders. The main pathway for mercury to humans is through the food chain and not by inhalation.
The main sources of mercury emissions in the UK are the manufacture of chlorine in mercury cells, non-ferrous metal production, coal combustion, and crematoria. UK emissions of mercury are uncertain and it is estimated that the range is from 13 to 36 tonnes per year (DERA). Emissions are estimated to have declined by around ¾’s between 1970-1998 (NAEI), mainly due to improved controls on mercury cells and their replacement, and the fall in coal use.
Whilst there has been a decline in the level of European emissions of mercury, emissions from outside of Europe have started to increase – increasing the level of ambient concentrations in the continent.
Small amounts of Nickel are needed by the human body to produce red blood cells, however, in excessive amounts, can become mildly toxic. Short-term overexposure to nickel is not known to cause any health problems, but long-term exposure can cause decreased body weight, heart and liver damage, and skin irritation. The EPA does not currently regulate nickel levels in drinking water. Nickel can accumulate in aquatic life, but its presence is not magnified along food chains.
Selenium is needed by humans and other animals in small amounts, but in larger amounts can cause damage to the nervous system, fatigue, and irritability. Selenium accumulates in living tissue, causing high selenium content in fish and other organisms, and causing greater health problems in humans over a lifetime of overexposure. These health problems include hair and fingernail loss, damage to kidney and liver tissue, damage to circulatory tissue, and more severe damage to the nervous system.
The most important disasters with heavy metals were:
If you have concerns about your level of heavy metals there is now a simple Hair Tissue Analysis that you can do to determine the number of heavy metals that are circulating in your blood, as well as a full mineral profile.
Reading this article, one can conclude that heavy metals are everywhere and very difficult to completely avoid these days – however careful we are with our food choices.
In order to prevent free radical damage from toxic metals, it is important to undertake a natural toxic metal detox. The question, is what is the best choice as there are so many detox products on the market?
It is best to eliminate heavy metals naturally using a proven toxic metal formulation such as HMD™.
We recommend you take the HMD™ Ultimate Detox Pack which contains the HMD™ (Heavy Metal Detox) as well as LAVAGE, a herbal drainage remedy, and organic CHLORELLA.
This can be used by all the family and there are also children’s dosages too.
Natural toxic metal detox using HMD™ – designed for eliminating heavy metals naturally!
HMD™ is a natural heavy metals detox formulation for removing heavy metals from the body!

Alkaline Detox Diet best body detox best detox kit best detox supplements best full body detox best heavy metal detox best heavy metal detox for adults best herbal detox best metal detox best thc detox kit body cleanse supplements body detoxification supplements Causes Arsenic Poisoning children and heavy metals chlorella detox Copper Toxicity in Drinking Water detox kit detox pack detox supplements detox tablets Does Sweating Really Release early symptoms of heavy metal toxicity full body detox kit hair mineral analysis heavy metal detox heavy metal detox kit heavy metals detox heavy metals in food Heavy Metals in Makeup Heavy Metals in US Foods HMD Heavy Metal Detox HMD Ultimate Detox Pack Natural Heavy Metal Detox natural supplement for aluminum ORGANIC CHLORELLA pure body detox pure body extra detox Sexual desire in women Sources of Copper Exposure Thallium Poisoning toxic metal detox Toxic metals and sex Toxic metals and women's sexual desire toxic metals in food ultimate detox

The Science of Heavy Metal Detox Natural Approaches to Cleansing Your Body In our modern world, exposure to heavy metals chlorella detox is practically unavoidable. Whether it’s through the food

Barium Toxicity: Sources, Health Effects, and Natural Detoxification Methods Barium is a naturally occurring heavy metal found in various minerals, water sources, and industrial materials. Although barium has certain industrial
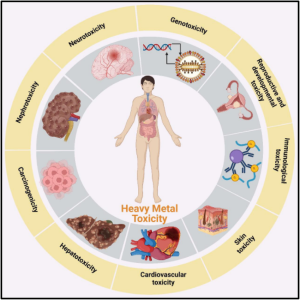
How Heavy Metals Are Stored in the Body Their Impact on Health Heavy metals chlorella detox such as lead, mercury, cadmium, arsenic, and aluminum are naturally occurring elements that can
Detoxmetals.com specializes in natural heavy metal detox supplements for eliminating heavy metals naturally from the body.
GMG DA VINCI HEALTH LTD Panayia Aimatousa 300 Aradippou 7101, Larnaca Cyprus
[sibwp_form id=1]
If you’re not receiving an email in your inbox, please check your spam folder. Sometimes legitimate emails can be mistakenly marked as spam. If you find the email there, you can mark it as “Not Spam” to ensure future messages from that sender go to your inbox
