
How Heavy Metals Can Disrupt Women’s Orgasms
How Heavy Metals Can Disrupt Women’s Orgasms And What You Can Do About It Let’s talk about something that doesn’t get talked about nearly enough: women’s orgasms—or the lack of
When people ask me why two clients can do “the same” heavy metal detox and get very different results, my short answer is: biology beats protocol. Your genes—especially the ones that handle binding, moving, and exporting metals—quietly determine how well chelators work in your body.
In this article, I’ll walk you through the key genetic polymorphisms that influence chelation efficacy, what the science actually shows, and how I translate this into practical, layperson-friendly personalization. I’ll also share where natural protocols like Dr. Georgiou’s HMD™ can fit, and how to think about “precision detox” without turning your life into a lab project.
Chelation isn’t just about swallowing a pill and “flushing” metals. It’s a three-step relay:
Genes can influence each step: how much metal gets mobilized, how sticky your internal binding proteins are, and how efficiently the kidneys and liver pump the metal (or metal-chelator complex) out.
In practice, I see four gene families come up again and again:
Let’s get specific.
1) Transporter genes: the “pumps” that make-or-break export
Most chelators aim to get metals into a form your kidneys can excrete. That last mile depends on transporter proteins in kidney tubules and bile canaliculi.
Multiple human studies link ABCC2 (MRP2) polymorphisms to differences in urinary inorganic mercury (Hg²⁺) excretion—i.e., how effectively your body can pump Hg out once it’s bound or conjugated. Think of MRP2 as a gate: if your variant is less active, you may retain more mercury and respond more slowly to detox.
What this means for chelation: If MRP2 is a bottleneck, I go slower, support bile/kidney flow aggressively (hydration, fiber, bitters), and use gentler mobilization so we don’t “traffic jam” metals.
The chelator HMD uses renal organic anion transporters OAT1 and OAT3 to cross into kidney cells and facilitate excretion. If your OAT activity is low, HMD protocols may be less efficient (or need adjusting).
What this means: In people with signs of sluggish OAT function (or relevant polymorphisms), I often prioritize kidney support first, consider alternate dosing schedules, and sometimes favor chelators or binders that rely less on rapid OAT-mediated shuttling.
HMD concentrates in the renal cortex where most of the heavy metals are eliminated. If NaDC3 function varies, you could see different HMD handling and excretion—and therefore different response curves.
What this means: When someone “doesn’t move” much metal on HMD despite textbook dosing, I consider NaDC3/SLC13A3 variability plus the basics (hydration, urine pH, mineral status).
2) Glutathione and metallothionein genes: your internal “chelators”
Glutathione (GSH) conjugation is central to moving metals—especially methylmercury—toward excretion. Polymorphisms in GSTs (GSTM1/GSTT1 deletions and GSTP1 variants) and rate-limiting enzymes for GSH synthesis (GCLC/GCLM) have been associated with higher mercury levels and/or altered toxicity risk in cohorts and reviews. Practically, a GST-null genotype can mean slower conjugation and potentially a “slower chelation responder.”
What this means: I support GSH upstream (sulfur-rich foods; NAC; minerals like selenium, zinc) and move cautiously on mobilization. Sometimes I stagger chelators with phases that emphasize antioxidant repletion.
Metallothioneins are small, cysteine-rich proteins that bind metals like zinc, copper—and importantly cadmium and mercury—acting as rapid-response buffers. Reviews highlight MTs as dynamic “metal sponges,” and emerging data link MT gene variants (e.g., MT1/MT2) to differences in metal handling and toxicity risk. If your MTs are less responsive, mobilized metals may not be sequestered as safely inside cells while awaiting export.
What this means: I ensure adequate zinc/selenium, consider protein status, and keep mobilization mild at first. People with suspected MT vulnerabilities often do better with longer, lower-intensity detox phases.
3) Metal-specific genes: arsenic, lead, and mercury examples
Your liver converts inorganic arsenic into mono- and dimethylated forms for urinary excretion. Variants in AS3MT consistently predict how efficiently you methylate arsenic, which in turn affects body burden and risk. If methylation is inefficient, chelation can still help—but I’ll expect a slower decline in total arsenic unless we support one-carbon metabolism (folate, B12, choline, betaine).
ALAD (δ-aminolevulinic acid dehydratase) polymorphisms change how lead partitions between blood and bone. People with certain ALAD variants show different blood lead levels at similar exposures—and that can influence how much “drop” you see from a given chelation round because blood lead is the main thing chelators reduce first.
We’ve already met ABCC2 (MRP2) and the GSTs; together they shape mercury conjugation and export pathways. There’s also evidence that APOE genotype (notably ε4) may modify susceptibility to mercury neurotoxicity, which matters for how aggressively we mobilize mercury near the CNS. I’m more cautious with ε4 carriers: slower pacing, antioxidant emphasis, and making sure exit routes are wide open.
So… do these genes change how chelators work?
Short answer: yes, mechanistically—and often clinically—though the human chelator-by-genotype trials we all want are still scarce. Here’s how I think about the big agents:
Why genetics matter here too: HMD™ leans on natural mobilizers and binders (e.g., cilantro/coriander, chlorella growth factor and chlorella) and drainage support. Your GST/MT capacity and transporter activity still shape how smoothly metals move and exit. I’ve seen GST-null individuals benefit from a longer “priming” phase (sulfur foods, NAC, selenium, zinc) before ramping up; they generally report fewer detox symptoms and steadier progress.
Evidence context: The strongest genotype-chelation data exist for mercury excretion and transporter/GST variants, arsenic methylation (AS3MT), and lead kinetics (ALAD). While the HMD™ research base includes internal and practitioner reports, much of the genotype-specific literature comes from academic studies on pathways (MRP2/ABCC2, GSTs, AS3MT, etc.), not branded protocols.
I integrate that pathway science to personalize natural detox programs on a case-by-case basis.
You don’t need a $1,000 gene panel to get started. Here’s the framework I use:
Step 1 — Baseline “phenotype” checks (no genetics yet)
Step 2 — Choose the on-ramp based on risk & resilience
Step 3 — Add targeted genetics if progress stalls or risk is high
Step 4 — Pick the tool and pacing
Step 5 — Monitor signals that matter
I like HMD™ (with Organic Chlorella and LAVAGE support) as a foundation protocol when I want steady progress with minimal drama. If someone’s GST capacity looks limited (by history or genotype), I’ll run a longer preparatory phase (sulfur foods, NAC, selenium, zinc, magnesium) and introduce HMD™ slowly.
The normal adult dose of HMD is 45 drops x 3 times daily, but with people with chronic diseases I have been known to drop this to one drop x 3 times daily and increase by one drop daily until reaching the recommended dose.
If MRP2/ABCC2 looks like a pinch-point, I’m extra fussy about hydration, fiber, bitters, and bowel regularity so the exit routes don’t choke. The goal isn’t speed; it’s traction you can sustain.
(Note: formal genotype-by-HMD outcome studies haven’t been published to my knowledge; I’m applying pathway science—MRP2, GSTs, MTs, etc.—to personalize natural programs.)
Quick science snapshots you can trust
If chelation hasn’t worked for you—or worked but came with rough side effects—it doesn’t mean detox “isn’t your thing.” It probably means your biology needs a different rhythm:
I’m a big believer in precision detox: less heroics, more fit. Your genes don’t dictate your destiny—but they do tell us how to make chelation work with your body, not against it.
Disclaimer: This article is educational and not medical advice. Always work with a qualified clinician when using chelators, especially if you have kidney/liver disease, are pregnant/breastfeeding, or are on interacting medications.

Alkaline Detox Diet best body detox best detox kit best detox supplements best full body detox best heavy metal detox best heavy metal detox for adults best herbal detox best metal detox best thc detox kit body cleanse supplements body detoxification supplements Causes Arsenic Poisoning children and heavy metals chlorella detox Copper Toxicity in Drinking Water detox kit detox pack detox supplements detox tablets Does Sweating Really Release early symptoms of heavy metal toxicity full body detox kit hair mineral analysis heavy metal detox heavy metal detox kit heavy metals detox heavy metals in food Heavy Metals in Makeup Heavy Metals in US Foods HMD Heavy Metal Detox HMD Ultimate Detox Pack Natural Heavy Metal Detox natural supplement for aluminum ORGANIC CHLORELLA pure body detox pure body extra detox Sexual desire in women Sources of Copper Exposure Thallium Poisoning toxic metal detox Toxic metals and sex Toxic metals and women's sexual desire toxic metals in food ultimate detox

How Heavy Metals Can Disrupt Women’s Orgasms And What You Can Do About It Let’s talk about something that doesn’t get talked about nearly enough: women’s orgasms—or the lack of
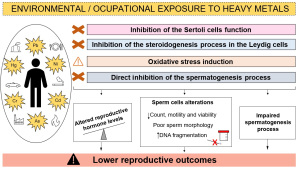
Men’s Sexual Health and Heavy Metals How Heavy Metals Wreak Havoc on Male Sexual Function Let’s get into the mechanics. Sexual health, especially things like getting and maintaining an erection,

How Heavy Metals Can Wreck a Woman’s Sexual Libido And What You Can Do About It Let’s talk about something that doesn’t often get enough attention: how environmental toxins, hefty
Detoxmetals.com specializes in natural heavy metal detox supplements for eliminating heavy metals naturally from the body.
GMG DA VINCI HEALTH LTD Panayia Aimatousa 300 Aradippou 7101, Larnaca Cyprus
[sibwp_form id=1]
If you’re not receiving an email in your inbox, please check your spam folder. Sometimes legitimate emails can be mistakenly marked as spam. If you find the email there, you can mark it as “Not Spam” to ensure future messages from that sender go to your inbox
