
Heavy Metals, Your Immune System, and Autoimmunity
Heavy Metals, Your Immune System, and Autoimmunity The Unseen Link Ever wondered why, despite eating well and cutting out junk, your body still feels off? Or why auto-inflammatory flare-ups don’t

There is a growing body of evidence that exposures to metals early in life (in utero and postnatal) increase the risk of developing adult diseases such as cancer, cardiovascular disease, non-alcoholic fatty liver disease, and diabetes.
Of particular concern is exposure to the metalloid arsenic, a drinking water contaminant and worldwide health concern.
Epidemiological studies of areas with high levels of arsenic in the drinking water, such as some regions in Chile and Bangladesh, indicate an association between in utero arsenic exposure and the development of adult diseases.
Therefore, the need for experimental models to address the mechanism underlining early onset of adult diseases have emerged including the in utero and whole-life exposure models.
This review will highlight the epidemiological events and subsequent novel experimental models implemented to study the impact of early life exposure to arsenic on the development of adult diseases.
Chronic exposure to arsenic through drinking water has the potential to cause adverse pregnancy outcomes, although the association has not been demonstrated conclusively. This cross-sectional study assessed the association between arsenic in drinking water and spontaneous abortion, stillbirth, and neonatal death.
In this cross-sectional study, 533 women were interviewed. Information on sociodemographic characteristics, drinking water use, and adverse pregnancy outcomes was obtained through a structured pretested interviewer-administered questionnaire. The respondents reported use of a total of 223 tube wells; for 208 wells, water samples were measured using an ultraviolet/visible spectrophotometry method, whereas 15 were measured by flow-injection hydride generation atomic absorption spectrometry (FIHG-AAS).
Excess risks for spontaneous abortion and stillbirth were observed among the participants chronically exposed to higher concentrations of arsenic in drinking water after adjusting for participant’s height, history of hypertension and diabetes, and (for neonatal death only) age at first pregnancy. Comparing exposure to arsenic concentration of greater than 50 μg/L with 50 μg/L or less, the odds ratios were 2.5 (95% confidence interval = 1.5–4.3) for spontaneous abortion, 2.5 (1.3–4.9) for stillbirth, and 1.8 (0.9–3.6) for neonatal death.
These study findings suggest that chronic arsenic exposure may increase the risk of fetal and infant death.
Arsenic contamination of groundwater has been reported from different parts of the world and is gradually evolving into a global problem.1–6 Bangladesh and West Bengal, a province of India, are the 2 most affected regions in the world.7,8 In Bangladesh, the main source of chronic arsenic exposure is through drinking contaminated groundwater. Until recently, groundwater has been the principal source of drinking water for more than 80% of the Bangladesh population.9 To control waterborne diseases such as cholera and diarrhea, millions of hand-pump tube wells were drilled, which inadvertently resulted in arsenic contamination because of the natural arsenic in the aquifer.10,11 This contamination threatens the attempts to provide safe drinking water to a larger population.7
Chronic exposure to arsenic is associated with cancer of the skin and internal organs and with several nonmalignant adverse health effects, including weakness, edema, conjunctival congestion, diabetes mellitus, hypertension, and respiratory conditions.12–18 Development of skin lesions and other conditions depends on the concentration and duration of arsenic exposure and possibly on interaction with nutritional and genetic factors.15
Although several studies have been conducted to determine the association between chronic arsenic exposure and adverse pregnancy outcome, the evidence remains inconclusive. Excess spontaneous abortion, stillbirth, and preterm birth rates among women with chronic arsenic exposure were first reported in Bangladesh in 2001.19 Ecologic studies in Chile, Sweden, Hungary, and Taiwan have also suggested associations between high arsenic exposure and spontaneous abortion, stillbirth, and preterm birth rates.20–23 Case-control studies from Massachusetts and Texas have shown weak associations between arsenic exposure and pregnancy outcomes.24,25
This cross-sectional survey included 533 women who were exposed to varying arsenic concentrations in their drinking water. We explore the risks of spontaneous abortion, stillbirths, and neonatal death among women consuming various concentrations of arsenic in their drinking water.
The study population was drawn from households in areas using tube wells with known arsenic concentrations. Areas were randomly selected from the unpublished database of NGO Forum for Drinking Water Supply & Sanitation on water quality monitoring. These areas were served by a total of 223 tube wells: 85 tube wells in 29 villages in the Comilla district and 2 villages in the Chandpur district (approximately 110 km from Dhaka, the capital city of Bangladesh), and 138 tube wells in 43 villages in the Chuadanga district (approximately 300 km from Dhaka). These districts are known to be affected by arsenic, according to the British Geological Survey report.11 The range of measured arsenic concentration in tube well water ranged from nondetectable to 1710 μg/L. We obtained ethical approval from the Environmental Science discipline’s Research Committee of The Khulna University.
After obtaining the arsenic information on the tube wells, we identified all households using the selected tube wells. All women living in these households were identified by interviewers, and their eligibility status was determined. Eligible participants were ever-married women ages 15–49 years at the time of recruitment with at least 1 prior pregnancy and no history of smoking; they had to have been living in the study area since their marriage and to have drunk water from the respective tube well before and during the pregnancy. The number of ineligible women for each category was not recorded, apart from 2 women who were excluded because they were smokers. A total of 540 eligible women were identified. Seven declined to participate, and so 533 women were interviewed in this study.
A structured pretested interviewer-administered questionnaire was used to collect information on sociodemographic variables, drinking water use, antenatal care, and adverse pregnancy outcomes (ie, spontaneous abortion, stillbirth, and neonatal death). Two trained interviewers (1 man and 1 woman) administered the questionnaires by face-to-face interview. These 2 interviewers were kept blind to the arsenic concentration of the tube wells to reduce interviewer bias, although complete blinding was not possible because most of the tube wells had previously been painted red or green to identify the presence or absence of arsenic contamination. The purpose of the interview was explained to the participants, and verbal consent was obtained before beginning each interview.
A natural failure of pregnancy within the first 28 weeks of gestation was regarded as spontaneous abortion.26 A stillbirth was considered to be any delivery after 28 completed weeks of gestation in which the baby did not breathe or show any sign of life.27 Neonatal death was defined as the death of the newborn within 28 days after birth.27
NGO Forum for Drinking Water Supply & Sanitation is the networking service delivery organization working in the water and sanitation sector in Bangladesh. As part of its regular water quality monitoring activities, this organization performs additional tests on 1% to 2% of the randomly selected tube well water samples that had been collected and tested earlier by the arsenic-detecting field kits. These additional tests were conducted for samples from different parts of the country at the Forum’s water quality testing laboratory using the ultraviolet/visible spectrophotometry method. A small proportion of these laboratory results are crosschecked with the flow-injection hydride generation atomic absorption spectrometry (FIHG-AAS) method at the laboratory of School of Environmental Studies, Jadavpur University, India.
A single well-water measurement was used to characterize chronic arsenic exposure for each study subject. We calculated duration of individual arsenic exposure from the period of use of the particular tube well. Arsenic concentration in 208 tube wells was measured using the ultraviolet/visible spectrophotometry method, whereas 15 were measured by flow-injection hydride generation atomic absorption spectrometry. The minimum detection level for arsenic is 30 μg/L by the first method and 3 μg/L by the second method.
We used logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for each outcome, initially adjusted for the potential confounders of participants’ education, age at marriage, age at first pregnancy, history of antenatal care, weight, height, age at first menstruation, duration of menstrual period, history of hypertension, diabetes, and vaccination during pregnancy. Covariates that were included in the final models were those that altered the odds ratio for the effect of arsenic concentration by more than 10%. For this purpose, arsenic concentration was dichotomized as 0 to 100 μg/L and >100 μg/L. Covariates included by this criterion were participant’s height, history of hypertension, and history of diabetes mellitus; for neonatal death, age at first pregnancy was also included. Only the first pregnancy outcome per woman was analyzed. Arsenic concentration was categorized as ≤50 μg/L and >50 μg/L, (and subcategorized as) 51–100 μg/L and >100 μg/L; for each, duration of exposure was categorized as up to 10 years and more than 10 years. Arsenic concentrations recorded as “zero” were replaced by the next smallest value in the data (30 μg/L) because the spectrophotometry method used cannot reliably discriminate below this concentration. Confidence intervals from these analyses were based on standard errors adjusted for clustering of outcomes among women using the same tube well using the Huber/White robust “sandwich” estimator of variance, which is based on the within-cluster correlation observed in the data.
Table 1 presents background characteristics, arsenic exposure, and pregnancy outcomes.

Results regarding the association of arsenic concentration and adverse pregnancy outcomes are given in Table 2. An increased risk of spontaneous abortion and stillbirth is observed for arsenic over 50 μg/L.

The combined effects of arsenic concentration and duration of exposure on pregnancy outcomes are presented in Table 3. For most levels of arsenic concentration, the risk estimates are higher with longer maternal exposure. The reference group is those who had concentrations not exceeding 50 μg/L regardless of the duration of exposure.

We assessed the risk of adverse pregnancy outcomes at several concentrations of arsenic in drinking water. The findings suggest an association between chronic arsenic exposure through drinking water and spontaneous abortion and stillbirth. The association between arsenic exposure and neonatal death was somewhat weaker than for the other pregnancy outcomes. This may be the result of the presence of related risk factors that were not considered in this study or may be because the biologic effects of arsenic occur early in fetal development.
Risks were generally higher for all 3 pregnancy outcomes with longer duration of arsenic exposure. Chronic arsenic exposure has been reported to be associated with adverse pregnancy outcomes in a few studies,19–24 although the evidence is only suggestive. A number of the studies conducted thus far are ecologic and therefore subject to additional potential biases, whereas some studies lacked information on several other risk factors associated with the exposure and outcomes.28
Animal model experimentation also exerted the toxicity of several forms of arsenic on pregnancy outcomes, including malformations.29 For the exposure measured in this study (ie, chronic arsenic exposure), both arsenite and arsenate exposures are relevant, because arsenate is converted to arsenite by glutathione during biomethylation in the human body.29 Arsenite has been observed to be more potent than arsenate.30 Recent studies reported that arsenic crosses the human placenta,31 although more than 90% of the arsenic in plasma and urine was in the form of dimethylarsinic acid, indicating an increase in the methylation of arsenic during pregnancy. Maternal toxicity has also been found to be associated with the adverse developmental effects of arsenic exposure. Maternal toxicity may in some instances be the causative factor in abnormal development of the embryos.32 This is probably the result of induction of metallothionein in the maternal liver that leads to a systemic redistribution of zinc and a transitory but developmentally adverse, zinc deficiency. These effects were produced in pregnant rats by arsenate. Exposure to arsenic also exerted direct adverse effects on explanted rodent embryos exposed to arsenic outside the maternal system. However, there was a poor correlation between maternal and developmental toxicity in an extensive literature analysis.33 Therefore, arsenic is likely to have direct toxic effects on embryos in vivo, but its effects might be exacerbated by external toxicity.28
There was no good documentation of pregnancy outcomes available in the study area, so we had to rely on the respondents when obtaining information. Hence, there is a chance of recall bias through differential recall of adverse pregnancy outcomes among those with higher exposure to arsenic if the respondent’s well was colorcoded correctly. Interviewer bias is less likely because the interviewers were unaware of the arsenic concentration levels of the subject’s usual drinking water source.
It would also have been desirable to have directly measured individual exposure data over time, because the available water samples reflected only a particular point in time and not the historical exposure. However, in absence of any reliable information on the past exposure, it was necessary to assume that arsenic concentration from the tube wells had been relatively constant over time. The historical consistency of arsenic concentration is of particular concern with shallow groundwater, which might be subject to greater fluctuation than water from a deeper well.28 Furthermore, we had information for only 1 well for each woman, and so her cumulative duration of arsenic exposure could not include exposure from other wells, although 1 of the eligibility criteria for study participation was having lived in the study area since their marriage. The amount of drinking water consumed was also not considered in this study. There could be variation in adverse pregnancy outcomes that is related to the amount of the exposed water the woman drank on average. Nevertheless, the strength of this study is the availability of individual arsenic exposure data and determination of risk at different arsenic concentration levels. Our data support the accumulating evidence that chronic arsenic exposure is associated with an increased risk of spontaneous abortion and stillbirth. Larger case-control studies are needed to confirm associations between arsenic and adverse pregnancy outcomes.
DOI:10.1080/19396368.2018.1480076
Syst Biol Reprod Med. 2018 Jun 6:1-15.

Alkaline Detox Diet best body detox best detox kit best detox supplements best full body detox best heavy metal detox best heavy metal detox for adults best herbal detox best metal detox best thc detox kit body cleanse supplements body detoxification supplements Causes Arsenic Poisoning children and heavy metals chlorella detox Copper Toxicity in Drinking Water detox kit detox pack detox supplements detox tablets Does Sweating Really Release early symptoms of heavy metal toxicity full body detox kit hair mineral analysis heavy metal detox heavy metal detox kit heavy metals detox heavy metals in food Heavy Metals in Makeup Heavy Metals in US Foods HMD Heavy Metal Detox HMD Ultimate Detox Pack Natural Heavy Metal Detox natural supplement for aluminum ORGANIC CHLORELLA pure body detox pure body extra detox Sexual desire in women Sources of Copper Exposure Thallium Poisoning toxic metal detox Toxic metals and sex Toxic metals and women's sexual desire toxic metals in food ultimate detox

Heavy Metals, Your Immune System, and Autoimmunity The Unseen Link Ever wondered why, despite eating well and cutting out junk, your body still feels off? Or why auto-inflammatory flare-ups don’t
Wearable Sweat-Induction Devices for Detox What Works, What Doesn’t, and How I Combine Them with Dr. Georgiou’s HMD™ Protocol When people ask me for a “do-able” detox routine that doesn’t
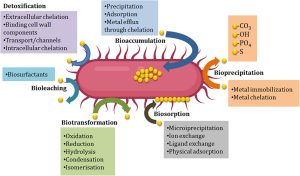
The Microbiome’s Role in Heavy-Metal Absorption and Elimination Why your gut bacteria can make detox easier—or harder (and how HMD™ fits in) If two people eat the same fish, drink
Detoxmetals.com specializes in natural heavy metal detox supplements for eliminating heavy metals naturally from the body.
GMG DA VINCI HEALTH LTD Panayia Aimatousa 300 Aradippou 7101, Larnaca Cyprus
[sibwp_form id=1]
If you’re not receiving an email in your inbox, please check your spam folder. Sometimes legitimate emails can be mistakenly marked as spam. If you find the email there, you can mark it as “Not Spam” to ensure future messages from that sender go to your inbox
